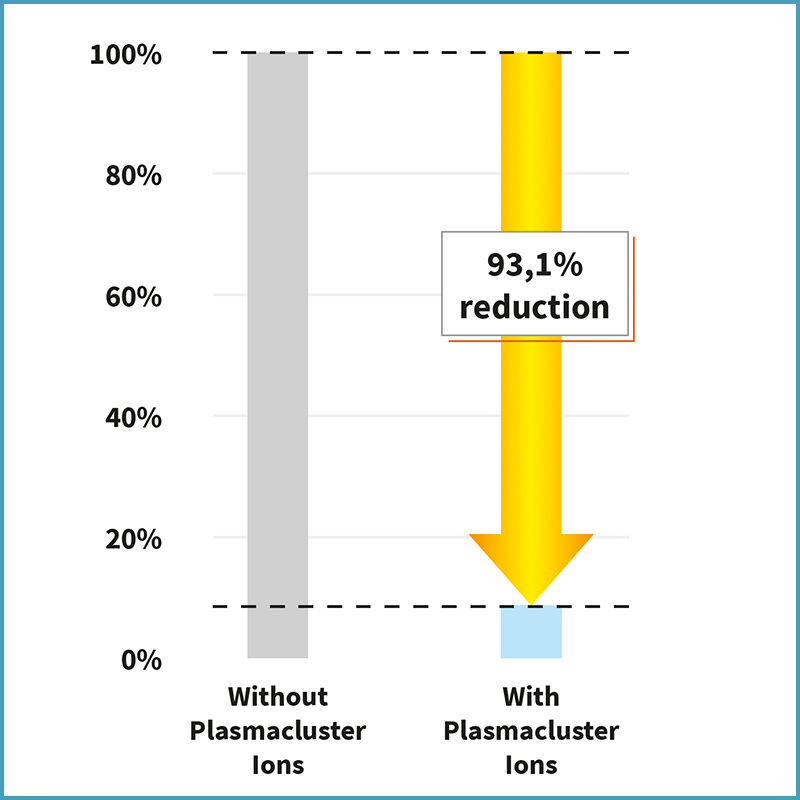
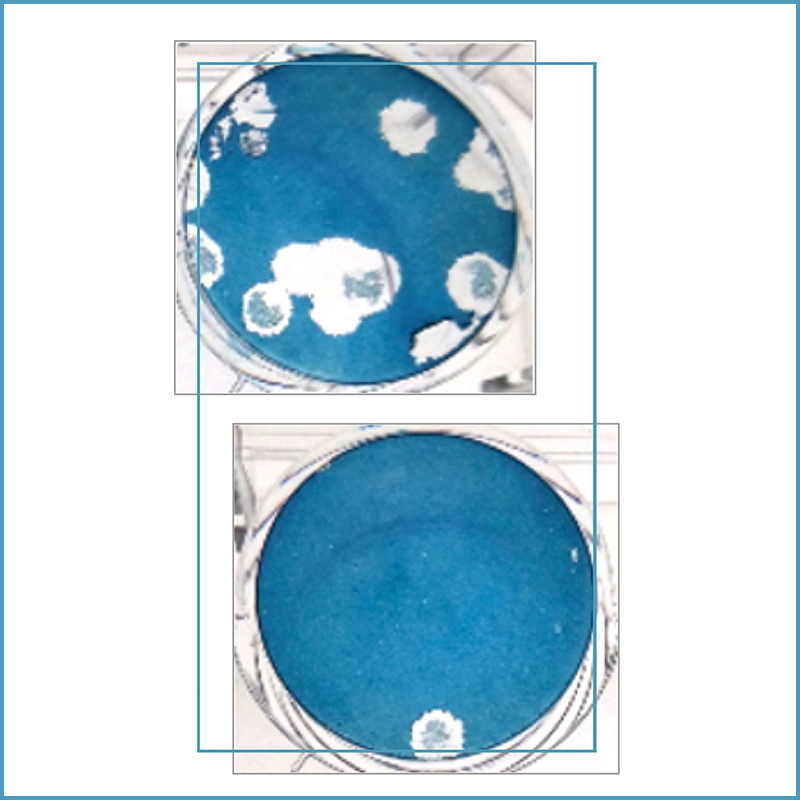
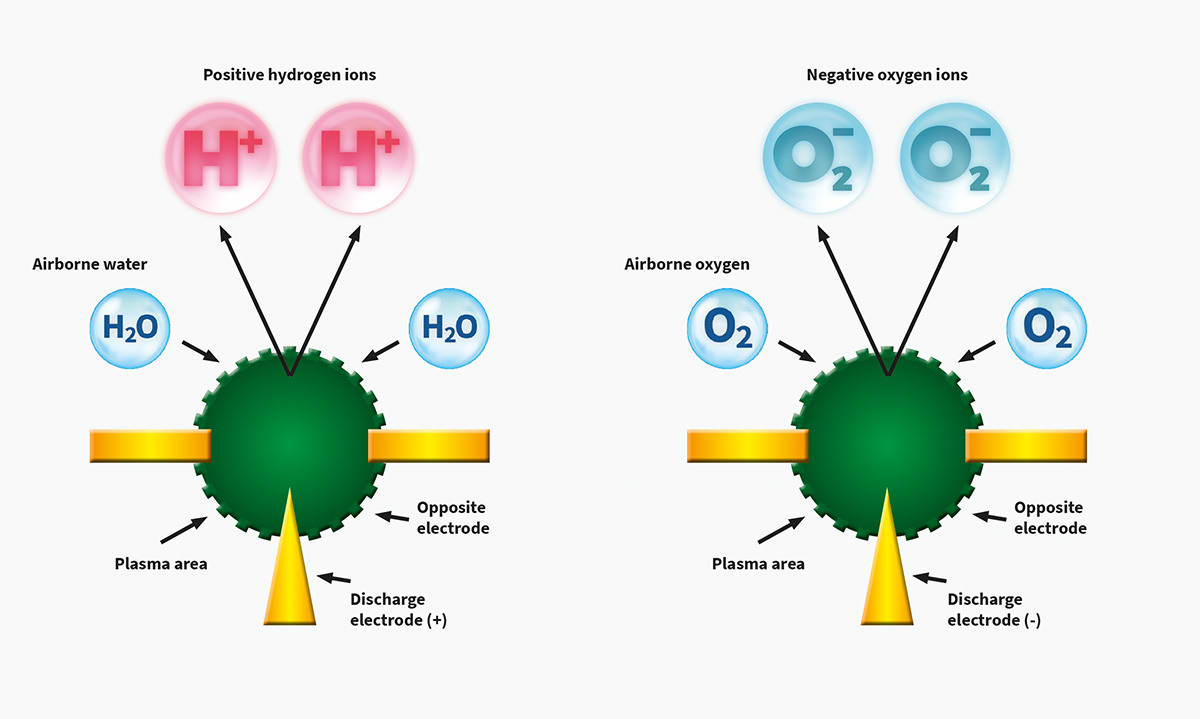
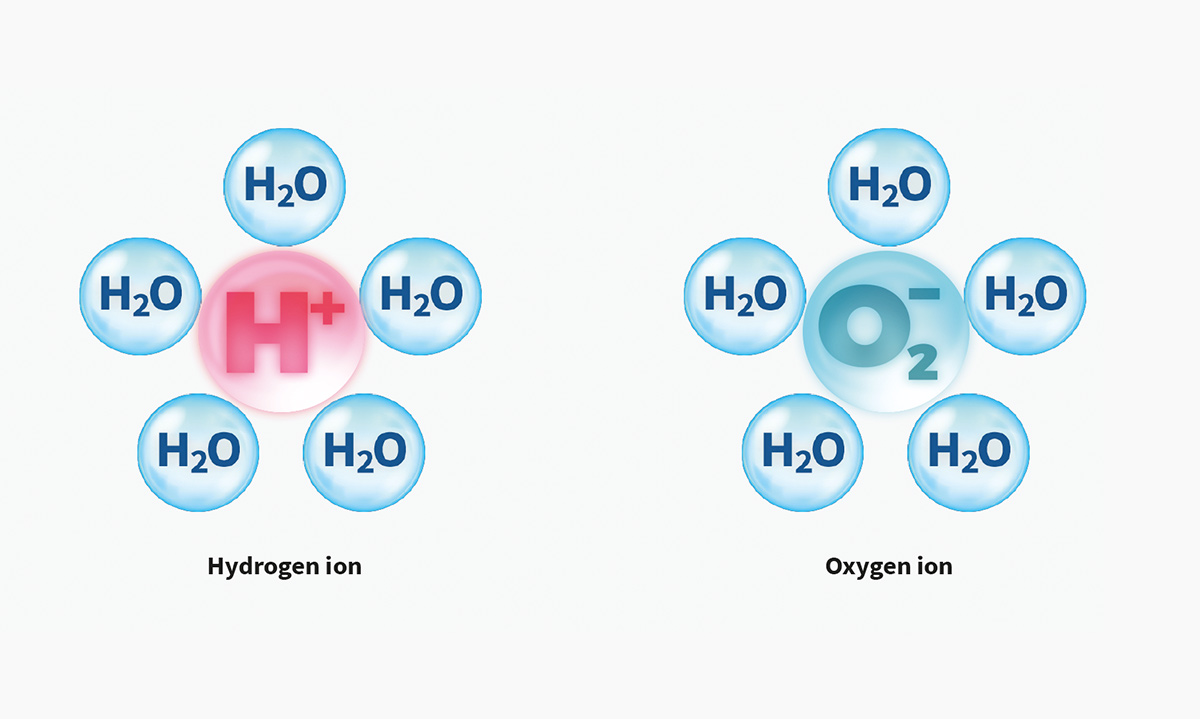
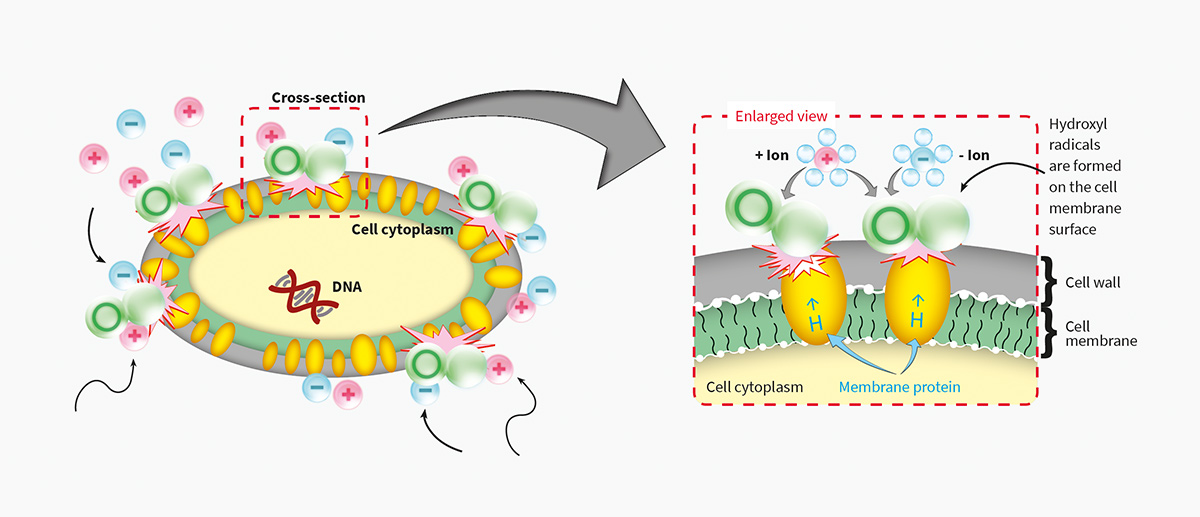
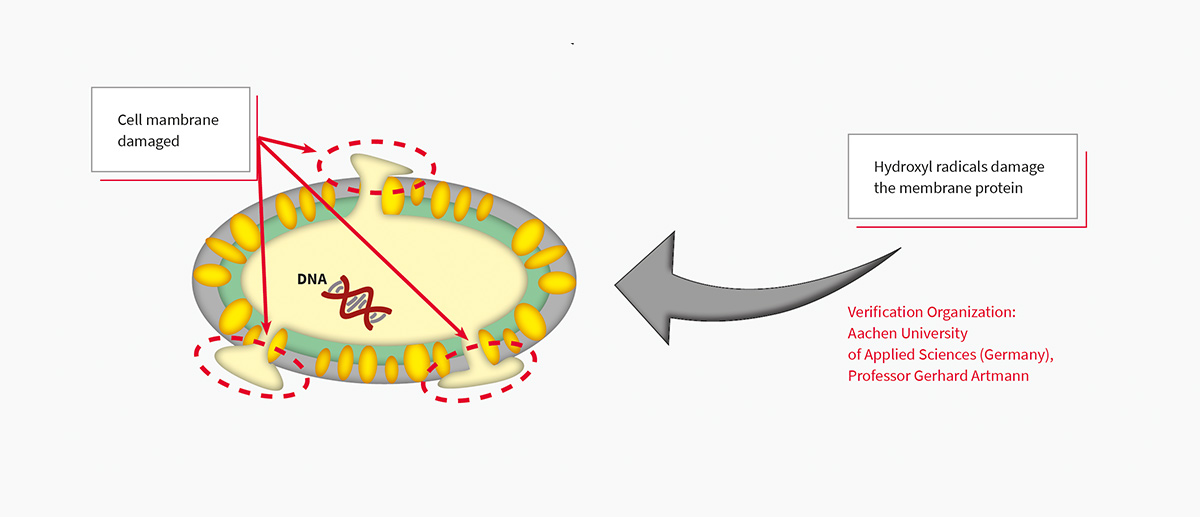
Plasmacluster Technology Demonstrates Effectiveness in Reducing Airborne Novel Coronavirus (SARS-CoV-2)*1, a World First*2

In December 2019,
an outbreak of “Coronavirus disease 2019 (COVID-19)” caused by a novel coronavirus (SARS-CoV-2) was reported, and by August 2020, more than 25 million people have been infected with SARS-CoV-2 and more than 840,000 individuals died of this infectious disease in a world*5. This outbreak represents an urgent problem facing society, and demands that immediate countermeasures be taken across a wide range of fields.
In 2004,
Sharp demonstrated the effectiveness of Plasmacluster technology against feline (cat) coronavirus, a member of the Coronaviridae family*6. In the following year, 2005, Sharp also demonstrated its effectiveness against the original SARS coronavirus*7 (SARS-CoV), which caused the outbreak (2002-2003) and genetically similar to the novel coronavirus (SARS-CoV-2). Now, Sharp has demonstrated its effectiveness against SARS-CoV-2 in airbornedroplets.
Since 2000,
Sharp has promoted academic marketing*8 to demonstrate the effectiveness of Plasmacluster technology, working in collaboratio with independent third-party research organizations around the world. Thus far, numerous independent research organizations have proven its clinical efficacy in suppressing the activity of harmful substances including new pandemic influenza viruses, drug-resistant bacteria, and mite allergens, as well as in reducing bronchial inflammation levels*9 in children with asthma. At the same time, the safety of Plasmacluster ions has also been confirmed*10. Sharp will continue to contribute to society by conducting a wide range of studies demonstrating the effectiveness of Plasmacluster technology.

 Preporučeno
Preporučeno Veličina ekrana
Veličina ekrana Tehnologija
Tehnologija TV rešenje
TV rešenje Rezolucija
Rezolucija Dodaci
Dodaci